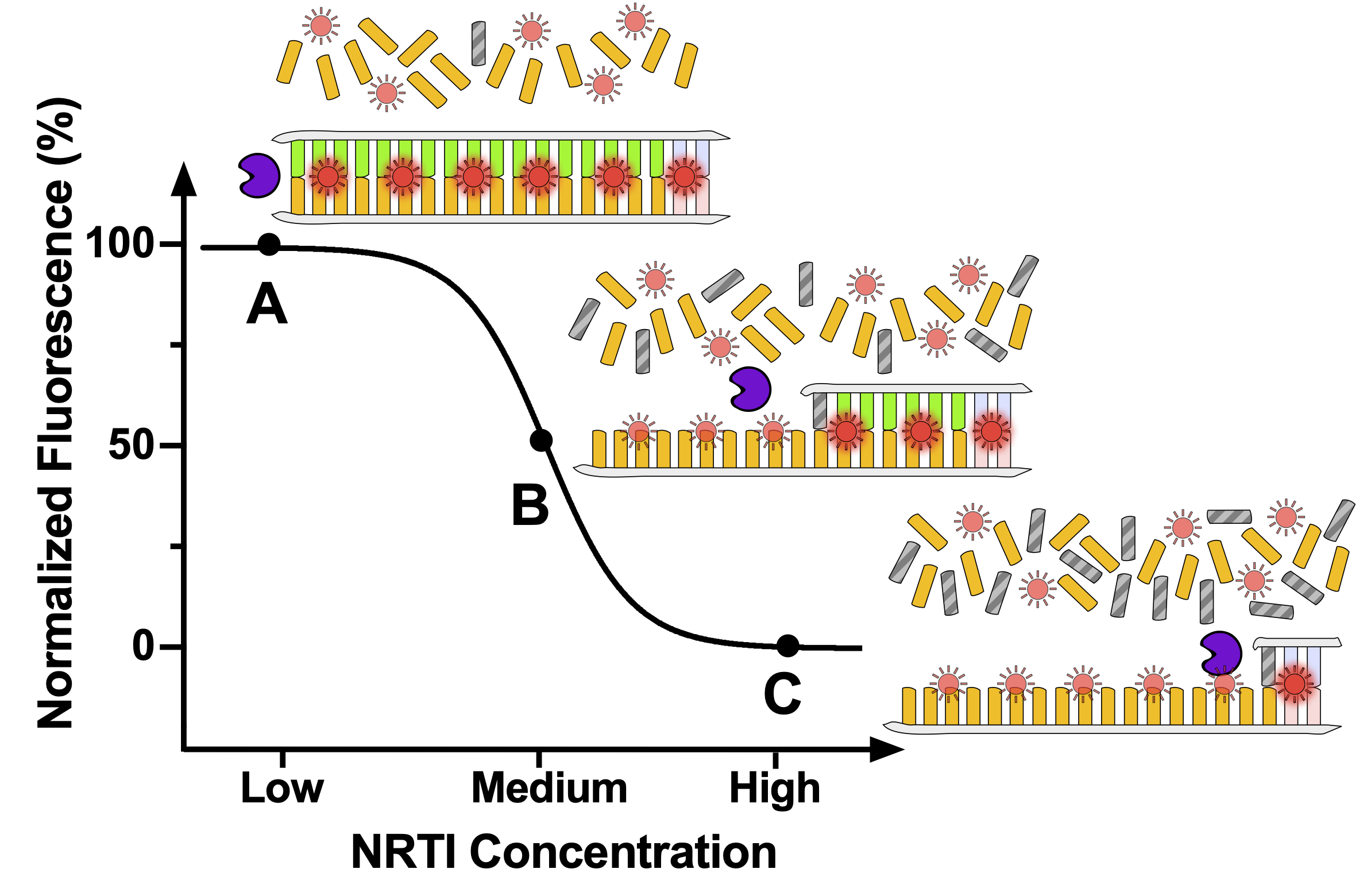
Poor adherence to pre-exposure prophylaxis (PrEP) and antiretroviral therapy (ART) can lead to human immunodeficiency virus (HIV) acquisition and emergence of drug resistant infections, respectively. Measurement of antiviral drug levels provides objective adherence information that may help prevent adverse health outcomes. Gold standard drug-level measurement by liquid chromatography/mass spectrometry is centralized, heavily instrumented, and expensive and is thus unsuitable and unavailable for routine use in clinical settings. We developed an innovative, simple assay to measure HIV drug levels. The assay measures the inhibition of reverse transcriptase, the enzyme responsible for HIV DNA synthesis, which is directly correlated with the levels and activity of tenofovir-diphosphate. The assay uses a novel, custom designed DNA template, a well-characterized mathematical model, and intercalating fluorescence dyes to objectively measure long-term PrEP adherence. Our test is suitable for routine use in clinical laboratories and could be integrated into a format for point-of-care (POC) testing in a clinic or for use in a patient’s home as a self-test.
Representative Publications
1. Olanrewaju, A. O.; Sullivan, B. P.; Zhang, J. Y.; Bender, A. T.; Sevenler, D.; Lo, T. J.; Fernandez-Suarez, M.; Drain, P. K.; Posner, J. D. (2019) An Enzymatic Assay to Measure Long-Term Adherence to Pre- Exposure Prophylaxis and Antiretroviral Therapy. bioRxiv, 832410. https://doi.org/10.1101/832410
2. Olanrewaju AO, Sullivan B, Bender AT, Zhang JY, Lo TJ, Bardon AR, Stekler JD, Drain PK, Posner JD. (2019) Development of an enzymatic assay for quantitative measurement of adherence to antiretroviral therapy and pre-exposure prophylaxis. The 14th International Conference on HIV Treatment and Prevention Adherence, Miami, FL.